Novel Synthesis builds customized materials for energy storage and conversion
Solid State Thin Films Deposited by Pulsed Laser Deposition
 Synthesized thin film lithium ion battery materials present many advantages for characterization and performance over the conventional slurry electrodes made for coin cell batteries. The absence of conductive and binder additives facilitates the direct reaction with the active material. This is crucial in surface characterization techniques where additives could convolute the signal from the active material. Solid state battery full cells also offer advantages over liquid cells, in safety and energy density. Unlike liquid organic electrolytes, solid state electrolytes used in solid state batteries are not sensitive to flammable conditions. Advanced analytical characterizations like in-situ TEM are enabled by solid state batteries. The small dimensions of the components and solid nature ease the fabrication of nano-batteries that can be observed under TEM. Therefore, solid state thin films are of great interest in fundamental studies of lithium ion battery materials which can be used in various applications. Our thin films are deposited with a Lambda Physik LPX-Pro 210 laser along with a high vacuum chamber with a substrate heater.
Synthesized thin film lithium ion battery materials present many advantages for characterization and performance over the conventional slurry electrodes made for coin cell batteries. The absence of conductive and binder additives facilitates the direct reaction with the active material. This is crucial in surface characterization techniques where additives could convolute the signal from the active material. Solid state battery full cells also offer advantages over liquid cells, in safety and energy density. Unlike liquid organic electrolytes, solid state electrolytes used in solid state batteries are not sensitive to flammable conditions. Advanced analytical characterizations like in-situ TEM are enabled by solid state batteries. The small dimensions of the components and solid nature ease the fabrication of nano-batteries that can be observed under TEM. Therefore, solid state thin films are of great interest in fundamental studies of lithium ion battery materials which can be used in various applications. Our thin films are deposited with a Lambda Physik LPX-Pro 210 laser along with a high vacuum chamber with a substrate heater.
Hydrothermal Synthesis
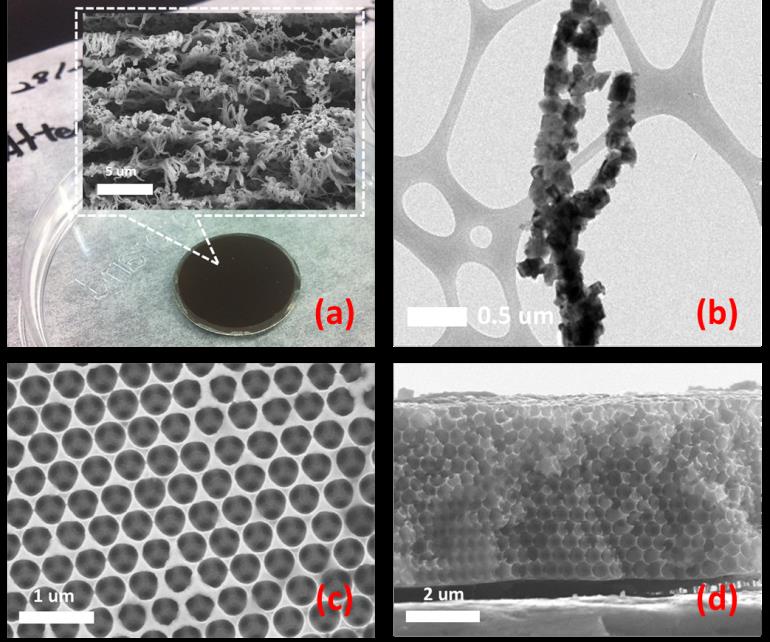
 Hydrothermal synthesis can be defined as a method of synthesis of crystal nucleation which is dependent on the solubility of the precursors under high temperature and pressure. The temperature gradient is maintained inside a Teflon container, where at a high temperature regime the solute dissolves and at a low temperature regime crystal is deposited which can be grown to the desired size. This is particularly suitable for the synthesis of crystallized particles with different morphologies. As shown in the figure: (a, b) spherical secondary particles, (c, d) square secondary particles. LESC utilizes this method to synthesize cathode materials for lithium ion batteries. Different cathode material morphologies can be synthesized to improve the electrochemical performance of a battery. LESC can also utilize specific tools (in advanced characterization) to further optimize battery electrodes synthesized using the hydrothermal process.
Hydrothermal synthesis can be defined as a method of synthesis of crystal nucleation which is dependent on the solubility of the precursors under high temperature and pressure. The temperature gradient is maintained inside a Teflon container, where at a high temperature regime the solute dissolves and at a low temperature regime crystal is deposited which can be grown to the desired size. This is particularly suitable for the synthesis of crystallized particles with different morphologies. As shown in the figure: (a, b) spherical secondary particles, (c, d) square secondary particles. LESC utilizes this method to synthesize cathode materials for lithium ion batteries. Different cathode material morphologies can be synthesized to improve the electrochemical performance of a battery. LESC can also utilize specific tools (in advanced characterization) to further optimize battery electrodes synthesized using the hydrothermal process.
The Polyol Process
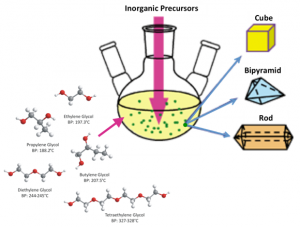 Among the chemical, physical, or electrochemical process generally used in particles production the polyol synthesis of inorganic nanoparticles is simple and versatile. The polyol process allows one to control the nucleation and growth steps in specific media. This process yields particles with well-defined characteristics, uniform shape, submicron or nanometer range with a narrow size distribution, and with a low degree of agglomeration.
Among the chemical, physical, or electrochemical process generally used in particles production the polyol synthesis of inorganic nanoparticles is simple and versatile. The polyol process allows one to control the nucleation and growth steps in specific media. This process yields particles with well-defined characteristics, uniform shape, submicron or nanometer range with a narrow size distribution, and with a low degree of agglomeration.
This is a facile route for preparing finely divided metal powders by reduction of inorganic precursors in liquid polyols. The solid precursor is suspended in the liquid polyol, which may be quite soluble (nitrate, chloride, acetate) or only slightly soluble (oxide, hydroxide). The solution or the suspension is stirred and heated at a specific temperature which reaches the boiling point of the polyol. Using this method of synthesis has several advantages such as high permittivity, mild reducing power conditions, and good chelating properties.
Nano-Structured Electrode Fabrication
Nano-structured materials for lithium-ion batteries have various advantages compared to the conventional micrometer-sized particles. The short distances for the lithium-ion transport within the materials increases significantly the rate of (de)intercalation. The characteristic time constant for diffusion is given by t=L2/D, where L is the diffusion length and D the diffusion constant. The time t for intercalation decreases with the square of the particle size on replacing micrometer with nanometer materials. In addition, a high surface area permits a high contact area with the electrolyte and hence a high lithium-ion flux across the interface. Electron transport with the materials is also enhanced by nanometer-sized materials. With the help of sol-gel based template synthesis, LESC can successfully fabricate the one-dimensional nano-structured cathode electrodes (a,b). The template-assisted electrodepostion with a self-assembled opal template also enable to obtain the three-dimensional nano-structured anode electrodes (c,d). LESC utilizes these synthesis methods to study the quantitative understanding of the effect of the nano-structural geometry on the elementary reaction steps involed in their electrochemical operation.
HIGHLIGHTED PUBLICATIONS
K. Carroll, M. Yang, G. M. Veith, N. J. Dudney, Y. S. Meng, “Intrinsic surface stability in LiMn2-xNixO4-δ (x=0.45, x=0.5) high voltage spinel materials for lithium ion batteries“, Electrochemical and Solid State Letters, 2012, 15(5), A72
H. Xia, Y. S. Meng, M. O. Lai, L. Lu, “Structural and Electrochemical Properties of LiNi0.5Mn0.5O2 Thin-Film Electrodes Prepared by Pulsed Laser Deposition“, Journal of the Electrochemical Society, 2010, 157 (3), A348
H. Xia, Y. S. Meng, M. O. Lai, L. Lu, “Structural and Electrochemical Properties of LiNi0.5Mn0.5O2 Thin-Film Electrodes Prepared by Pulsed Laser Deposition“, Journal of the Electrochemical Society, 2010, 157 (3), A348
H.-M. Cho, Y. S. Meng, “Effect of Ni/Mn ordering on elementary polarizations of LiNi0.5Mn1.5O4 spinel and its nanostructured electrode“, Journal of the Electrochemical Society, 2013, 160(9), A1482
Advanced Characterization provides insightful understanding of nanomaterial.
X-ray Photoelectron Spectroscopy
Quasi in-situ X-ray photoelectron spectroscopy (XPS) is utilized to examine surface transformations of the cathode electrode during electrochemical cycling. LESC utilizes a quasi in-situ setup where samples can be charged to different states and analyzed without air exposure.XPS can provide information on elemental identification, chemical state of element, and relative composition of constituents in surface region. This is critical for the development of new battery materials.
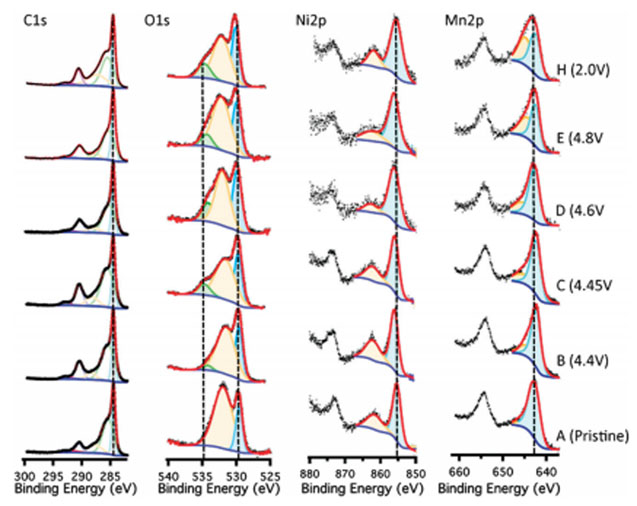
This figure shows the resulting XPS spectra for the C1s, O1s, Mn2p and Ni2p region scans at various points during the first electrochemical charge and discharge. For example,the pristine Ni2p shows a binding energy of 855 eV. The peak shifts to a higher binding energy of 856.5 eV when fully charged, suggesting that the nickel oxidation state change is complete. Upon discharge the peak shifts back to the Ni2+.
Neutron Diffraction
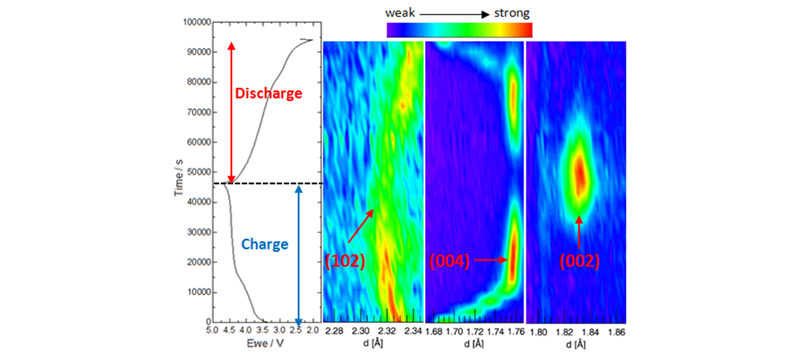
In-situ neutron diffraction (ND) has been developed to study the crystal structure evolution of both the cathode and the anode in a full cell during electrochemical cycling. The advantages of neutron diffraction: 1) high penetration enables simultaneous observation of cathode and anode during charge and discharge; 2) high sensitivity to light elements, lithium and oxygen; and 3) high contrast to distinguish TM ions. With the help of in-situ neutron diffraction, we can observe the phase transformation of graphite, lithium intercalation mechanism and oxygen evolution of the cathode material.
(a): 1st cycle charge/discharge curve of Lithium-rich / C cell and surface contour plots of (b): Lithium-rich (102); (c): LixC6 (004); (d): LiC6 (002) Neutron Diffractions.
Synchrotron X-ray Diffraction
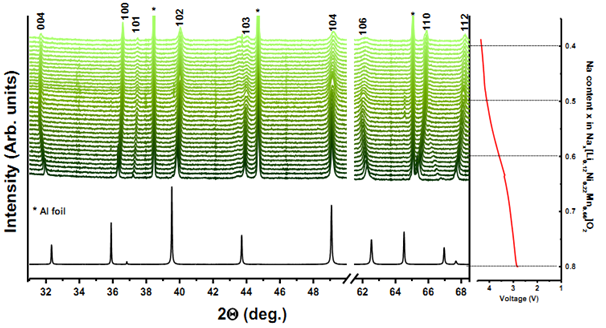 Due to the merits of high brightness and high intensity, high level of polarization and wide tunability in energy, synchrotron radiation technique provides an unique platform for analysis of the relationship among composition-structure-performance of materials for electrochemical system. Both ex-situ and in-situ X-ray Diffraction (XRD) data are collected by our group with a variety of electrode materials at Argonne National Lab on beamline 11-BM and at Brookhaven National Lab on beamline X14A respectively. The ex-situ studies of active materials involve arresting the electrochemical cycling at various stages and extracting the materials for characterization, so it may fail to capture short-lived metastable phases. The development of in-situ XRD with synchrotron sources further enables to monitor the phase evolution upon cycling and analyze the atomic site occupancy at the same time.
Due to the merits of high brightness and high intensity, high level of polarization and wide tunability in energy, synchrotron radiation technique provides an unique platform for analysis of the relationship among composition-structure-performance of materials for electrochemical system. Both ex-situ and in-situ X-ray Diffraction (XRD) data are collected by our group with a variety of electrode materials at Argonne National Lab on beamline 11-BM and at Brookhaven National Lab on beamline X14A respectively. The ex-situ studies of active materials involve arresting the electrochemical cycling at various stages and extracting the materials for characterization, so it may fail to capture short-lived metastable phases. The development of in-situ XRD with synchrotron sources further enables to monitor the phase evolution upon cycling and analyze the atomic site occupancy at the same time.
Scanning Transmission Electron Microscope and Electron Energy Loss Spectroscopy
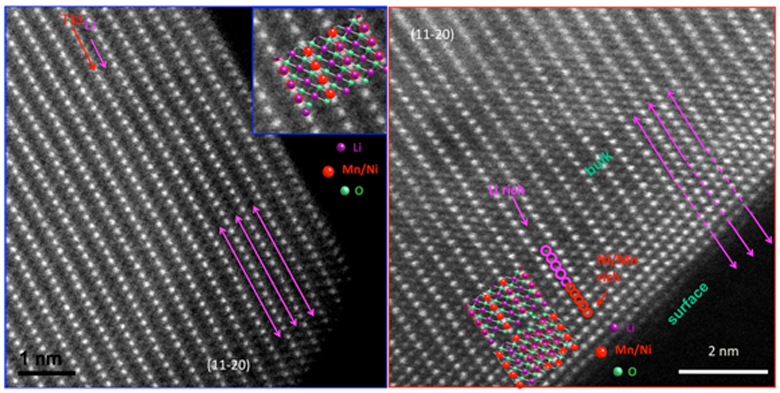 Scanning Transmission Electron Microscope and Electron Energy Loss Spectroscopy is a powerful tool that can obtain structural and chemical information with atomic resolution. The annular dark/bright field detector coupled with a spectrometer can simultaneously provide the local image and chemical information. LESC has applied this high-end technique to battery systems in ex-situ and in-situ, achieving both electrode and interface properties.
Scanning Transmission Electron Microscope and Electron Energy Loss Spectroscopy is a powerful tool that can obtain structural and chemical information with atomic resolution. The annular dark/bright field detector coupled with a spectrometer can simultaneously provide the local image and chemical information. LESC has applied this high-end technique to battery systems in ex-situ and in-situ, achieving both electrode and interface properties.
Coherent X-ray Diffractive Imaging
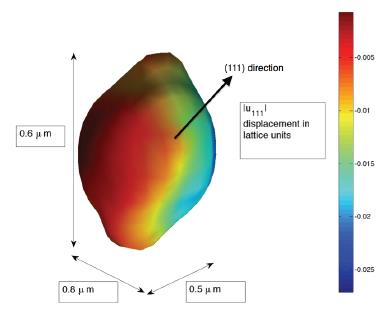
Coherent X-ray diffractive imaging (CXDI) is a three-dimensional structural analysis method for individual small crystals. A spatially coherent beam of X-rays traverse the sample, and produce the scattering from all parts of the sample, which can be expected to interfere in the far-field diffraction pattern. With the help of the computational phase retrieval algorithms, the inversion of the diffraction pattern yields a three-dimensional image of the density distribution and a projection of the strain within it. LESC utilizes this characterization to map the local three-dimensional strain inhomogeneity and electron density distribution of the battery materials in both ex-situ and in-situ methods. This will shed a new light on understanding the nano-mechanics and phase transformations during the battery operation.
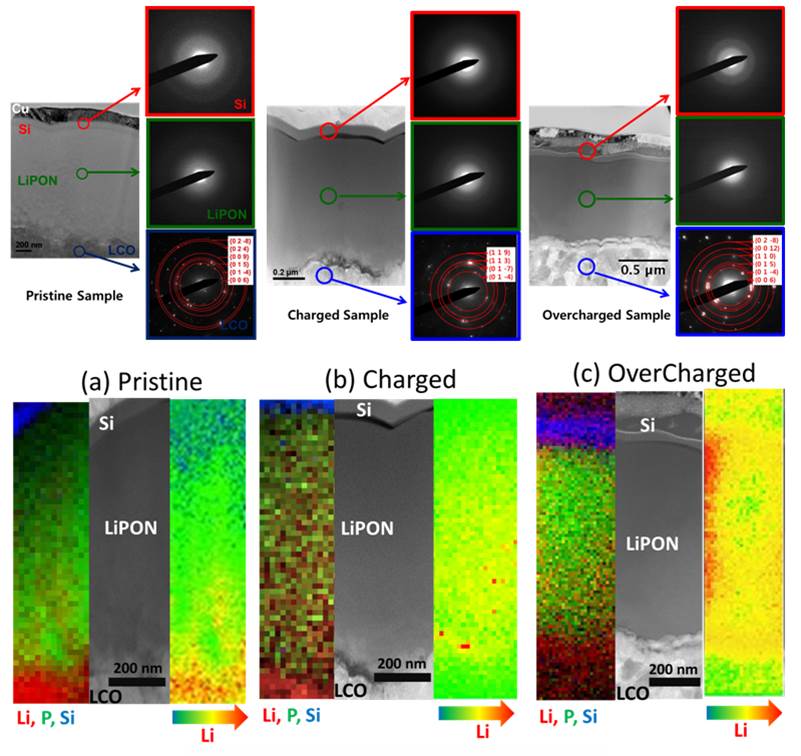
In-Situ Transmission Electron Microscope
HIGHLIGHTED PUBLICATIONS
H. D. Liu, C. R. Fell, K. An, L. Cai, Y. S. Meng, “In-situ neutron diffraction study of the xLi2MnO3-(1-x)LiMO2 (x=0, 0.5; M=Ni, Co, Mn) layered oxide compounds during electrochemical cycling“, Journal of Power Sources, 2013, 240, 772
J. Xu, D. H. Lee, R. J. Clement, X. Yu, M. Leskes, A. J. Pell,G. Pintacuda, X.-Q. Yang, C. P. Grey, Y. S. Meng, “Identifying the Critical Role of Li Substitution in P2-Nax[LiyNizMn1-y-z]O2″, Chem. Mater., 2014, 26, 1260
D. Santhanagopalan, D. Qian, T. Mcgilvary, Z. Wang, F. Wang, F. Camino, J. Graetz, N. Dudney, Y. S. Meng, “Interface Limited Lithium Transport in Solid-State Batteries“, J. Phys. Chem. Lett., 2014, 5, 298
A. Ulvestad, H. M. Cho, R. Harder, J. W. Kim, S. H. Dietze, E. Fohtung, Y. S. Meng, O. G. Shpyrko, “Nanoscale strain mapping in battery nanostructures“, Applied Physics Letters., 2014, 104(7), 073108
Computation and Simulation provide us with a map which can be applied to nanomaterials.
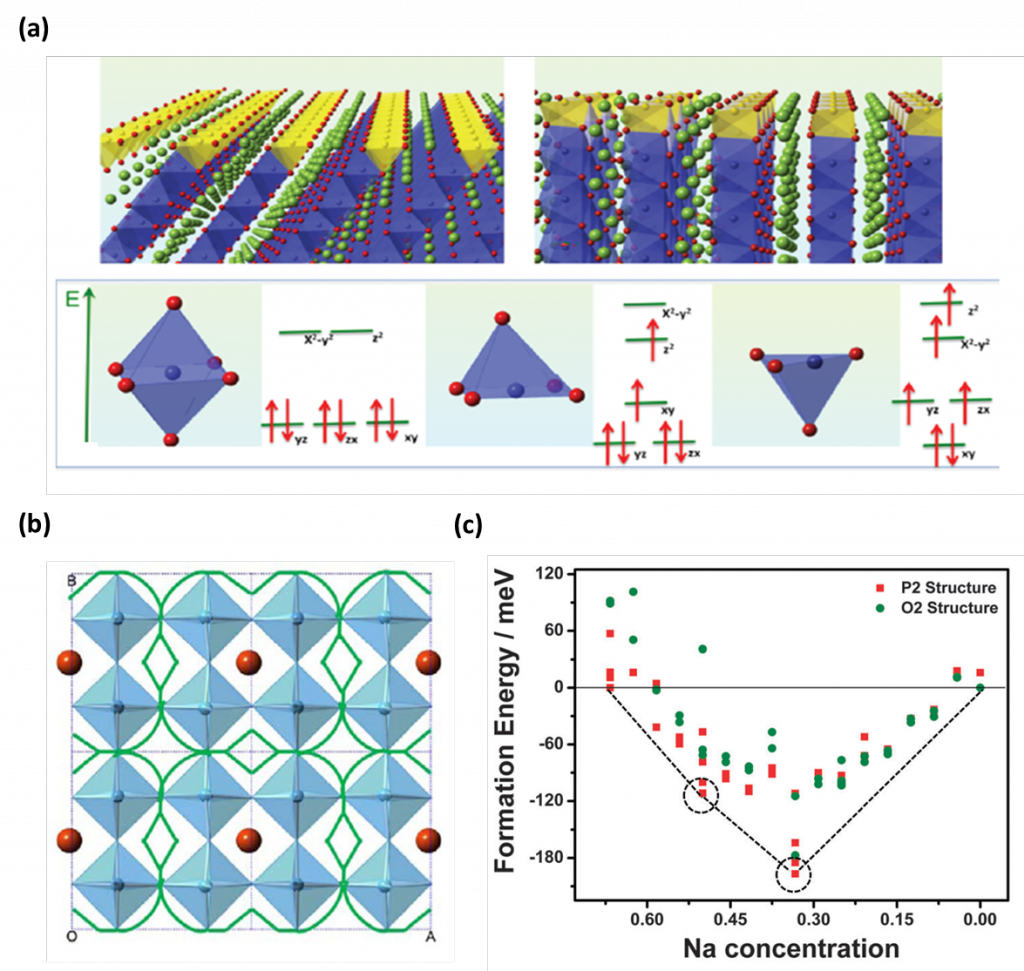
The important characteristics of rechargeable battery materials including electronic properties, ions path and their diffusivities, and the structural stability can be investigated state-of-the-art first principles calculations using VASP package. For example, Figure 1a. shows electronic spin transition from low spin to high spin in the surface of nanosized lithium cobalt oxide during (de)intercalation 1. This work suggests the significant criteria to develop high functional electrode materials by controlling the surfaces and interfaces of nano-sized materials. In addition, Li-ion diffusion path can be estimated by calculating its activation barriers suggesting Li-ion prefer a curved diffusion path to avoid empty sites as shown in Figure 1b 2. Thermodynamically the most stable structures in certain concentration of ions can also be estimated with calculated convex hull of formation energies as shown in Figure 1c 3. This is the direct measure of phase stability and identifies the intermediate phases during electrochemical reactions
Figure 1. (a) Electronic spin transition from low spin to high spin in the surface of nano-sized LiCoO2, (b) estimated Li diffusion path in La-poor layer, and (c) the formation energy convex hull (dotted line) for Nax[Ni1/3Mn2/3]O2 cells.
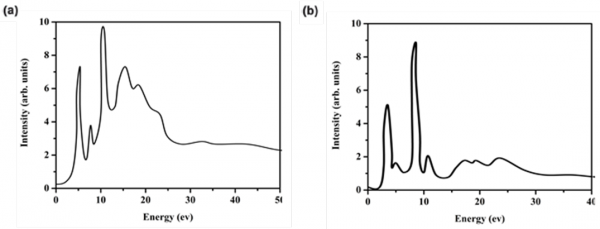
WEN2K package is also applied in our lab in order to fully understand the fine structures of electron energy loss spectroscopy (EELS). The key fact about first principles calculations WIEN2K package is that the core shell electrons and the valence electrons are treated distinguished, a linear combination of radial wave functions multiplied by spherical harmonics used for the core electrons, a plane wave expansion used for the valence electrons. This feature makes the WIEN2K give more accurate simulation of the EELS result compared with other computational package based on the pseudopotential method like VASP package as shown in Fig. 2. Our objective is to explore the detailed local electronic structures of lithium cathode materials, and simulate the EELS spectra based on the obtained density of states. We could also adopt the same theoretical and computational method to other more complex systems, such as core-shell structure and cathode materials with gradient structure.
Figure 2. (a) Lithium EELS K edge simulation using WIEN2K package for LiMn2O4, and (b) Li2O.
HIGHLIGHTED PUBLICATIONS
D. Qian, B. Xu, H.-M. Cho, T. Hatsukade, K. J. Carroll, Y. S. Meng, “Lithium Lanthanum Titanium Oxides: A Fast Ionic Conductive Coating for Lithium-Ion Battery Cathodes“, Chemistry of Materials, 2012, 24(14), 2744
D. Qian, Y. Hinuma, H. Chen, L-S Du, K. Carroll, G. Ceder, C. P. Grey, Y. S. Meng, “Electronic spin transition in nanosize stoichiometric lithium cobalt oxide“, Journal of the American Chemical Society, 2012, 134(14), 6096
D. H. Lee, J. Xu, Y. S. Meng, “An advanced cathode for Na-ion batteries with high rate and excellent structural stability“, Phys. Chem. Chem. Phys., 2013, 15, 3304