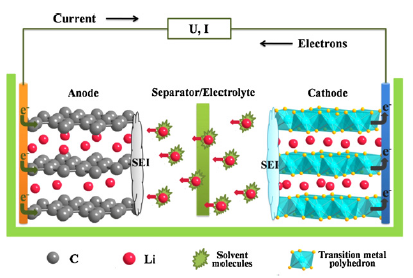
 Lithium ion batteries have become a key component of portable electronic devices as they offer high energy density, flexible lightweight design and a longer cycle life than other battery systems. More efficient batteries are required in the development of advanced transportation technologies in order to reduce the use of imported oil and the emission of greenhouse gas. Electrochemical energy storage has been identified as a critical enabling technology for advanced, fuel-efficient, light and heavy duty vehicles. New materials need to be designed to achieve higher energy/power densities, longer cycle lives and better reliability for such applications.
Lithium ion batteries have become a key component of portable electronic devices as they offer high energy density, flexible lightweight design and a longer cycle life than other battery systems. More efficient batteries are required in the development of advanced transportation technologies in order to reduce the use of imported oil and the emission of greenhouse gas. Electrochemical energy storage has been identified as a critical enabling technology for advanced, fuel-efficient, light and heavy duty vehicles. New materials need to be designed to achieve higher energy/power densities, longer cycle lives and better reliability for such applications.
Cathode
To enable LIB as the main on-board storage technology in plug-in hybrid electric vehicles (PHEVs) or electric vehicles (EVs), higher energy density materials such as the ‘‘Li-excess’’ layered oxides, formed as the composites between Li[Li1/3Mn2/3]O2 and LiMO2 (M = Ni, Mn, Co), are promising candidates as they offer much higher capacity (>250 mAh/g) and better safety with much reduced cost. Currently, the large first cycle irreversible capacity, low rate capacity and votage/capacity fading are the major problems in this series of materials. We are developing various characterization techniques (x-ray, neutron, electron based in-situ/ ex-situ techniques) and modeling methods (VASP, FEFF, Wen2k) to study the mechanism of these problems.
Anode
After nearly three and a half decades of optimization, the energy density (Wh kg-1) of Li-ion batteries is quickly approaching the intercalation chemistry’s upper limit threshold. Before transitioning to a totally new chemistry, one final improvement slated for commercial batteries involves replacing graphitic anodes with Si. Si’s theoretical capacity (Ah kg-1) is an order of magnitude larger than that of graphite’s, which translates to a final overall battery energy density increase of approximately 10 – 15%. Unfortunately, Si anodes are not yet ready for commercialization because designing reversible Si electrode structures has presented researchers with formidable engineering challenges.
The large irreversibility associated with the formative cycles of Si anodes is primarily due to mechanical pulverization and chemical instability. The mechanical pulverization of the Si active material has largely been solved by adopting nanostructures. Additionally, a wide range of electronically conductive binders and backend electrode coatings help to address the pulverization of the overall electrode structure. It is not yet clear how to create a stable solid electrolyte interphase (SEI) between Si and the organic liquid electrolyte in order to improve electrode Coulombic efficiency and reversibility.
Our group is interested in characterizing the composition of the SEI formed on amorphous-Si thin film electrodes fabricated by pulsed laser deposition, nano particles, and porous Si.
Highlighted Publications
K. Kang, Y.S. Meng, J. Breger, C.P. Grey and G. Ceder, “Electrodes with high power and high capacity for rechargeable lithium batteries“, Science, 2006, 311, 977
B. Xu, C. R. Fell, M. Chi, and Y. S. Meng, “Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study“, Energy & Environmental Science, 2011, 4, 2223
D. Qian, Y. Hinuma, H. Chen, L-S Du, K. Carroll, G. Ceder, C. P. Grey, Y. S. Meng, “Electronic spin transition in nanosize stoichiometric lithium cobalt oxide“, Journal of the American Chemical Society, 2012, 134(14), 6096
B. Xu, D. Qian, Z. Wang, Y. S. Meng, “Recent Progress in Cathode Materials Research for Advanced Lithium Ion Batteries“, Materials Science and Engineering R, 2012, 73(5), 51
Y. S. Meng, M. E. A.-d. Dompablo, “Recent Advances in First Principles Computatinal Research of Cathode Materials for Lithium-Ion Batteries“, Accounts of Chemical Research, 2012, 46(5), 1171
Sodium-ion Batteries
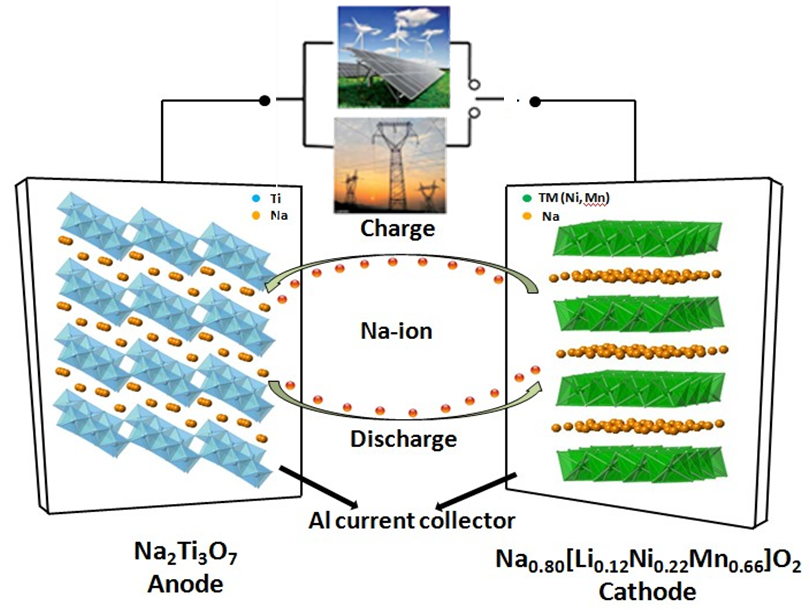 Na-ion batteries have recently gained increased recognition as intriguing candidates for next-generation large scale energy storage systems, owing to significant cost advantages stemming from the high natural abundance and broad distribution of Na resources. Although Na-ion battery materials are not comparable with their Li-ion counterparts which are one of the dominating energy technologies in this decade, there are studies suggesting that Na-ion systems should not be discarded.In particular, Na-ion batteries operating at room temperature could be suitable for applications where specific volumetric and gravimetric energy density requirements are not as stringent as in EVs, namely in electrical grid storage of intermittent energy produced via renewable sources. This would also contribute to a significant reduction of the costs connected to the use of renewable sources, which could then penetrate the energy market more easily and make Na-ion technology complementary to Li-ion batteries for stationary storage.
Na-ion batteries have recently gained increased recognition as intriguing candidates for next-generation large scale energy storage systems, owing to significant cost advantages stemming from the high natural abundance and broad distribution of Na resources. Although Na-ion battery materials are not comparable with their Li-ion counterparts which are one of the dominating energy technologies in this decade, there are studies suggesting that Na-ion systems should not be discarded.In particular, Na-ion batteries operating at room temperature could be suitable for applications where specific volumetric and gravimetric energy density requirements are not as stringent as in EVs, namely in electrical grid storage of intermittent energy produced via renewable sources. This would also contribute to a significant reduction of the costs connected to the use of renewable sources, which could then penetrate the energy market more easily and make Na-ion technology complementary to Li-ion batteries for stationary storage.
In our current research, both advance cathode and anode materials are developed and studied. In the cathode, Li-substituted layered P2−Na0.80[Li0.12Ni0.22Mn0.66]-O2 is brought out and investigated by advanced techniques such as neutron diffraction and nuclear magnetic resonance (NMR) spectroscopy. In the anode, we combine the first principle calculation and X-ray absorption spectroscopy to unveil the intercalation mechanism of Na2Ti3O7. High capacity and power density Na-ion full cell has also been achieved in our lab.
We are aiming at discovering high performance materials as well as understanding the energy storage mechanism, which are crucial for the development of Na-ion battery.
Flow Batteries
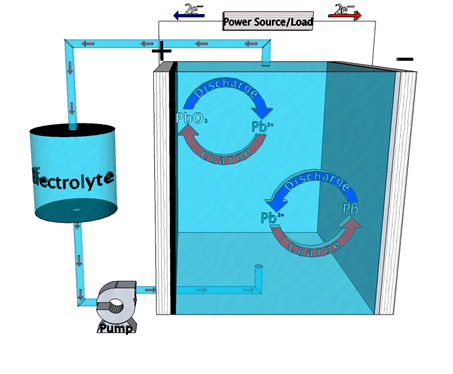 Among other low cost energy storage alternatives to Li-ion batteries, the Meng group explores several flow battery chemistries. Chief among them has been the soluble lead flow battery (SLFB). Social perception of lead-based products is extremely negative due to their association with an array of adverse health effects. The traditional lead-acid battery is the most highly recycled product on earth, however, trumping tires, paper, aluminum cans, and glass. Through centuries of use, we have had time to very thoroughly discern the harmful impacts of these materials, as well as methods to circumvent those negative aspects. A vast infrastructure currently exists to manufacture and sustainably maintain lead-acid batteries, arguably making them the cheapest and most realistic option to implement into grid-level energy storage today, without perverting the market with unsustainable government subsidy.
Among other low cost energy storage alternatives to Li-ion batteries, the Meng group explores several flow battery chemistries. Chief among them has been the soluble lead flow battery (SLFB). Social perception of lead-based products is extremely negative due to their association with an array of adverse health effects. The traditional lead-acid battery is the most highly recycled product on earth, however, trumping tires, paper, aluminum cans, and glass. Through centuries of use, we have had time to very thoroughly discern the harmful impacts of these materials, as well as methods to circumvent those negative aspects. A vast infrastructure currently exists to manufacture and sustainably maintain lead-acid batteries, arguably making them the cheapest and most realistic option to implement into grid-level energy storage today, without perverting the market with unsustainable government subsidy.
Much like the traditional lead-acid battery, SLFB chemistry incorporates the reaction of lead (Pb) and lead oxide (PbO2) at anode and cathode, respectively. Unlike traditional lead-acid, however, no sulfuric acid is used in the electrolyte. No sulfate-based products are generated, therefore, overcoming a major deterioration mechanism. By flowing electrolyte through the battery during operation, power and energy components are separated, making large-scale design efficient and flexible. Compared to other flow battery technologies SLFBs provide the added advantage of having a single electrolyte and no separator. Balance of plant is, therefore, considerably less expensive than other chemistries, including its traditional lead-acid predecessor. While the price point is markedly better than competing technologies, performance is not. Power density and cycle life in particular are much lower than technologies such as vanadium-redox. Our group has made progress improving upon these shortcomings, but current research aims to get better still.1
Solid State Batteries
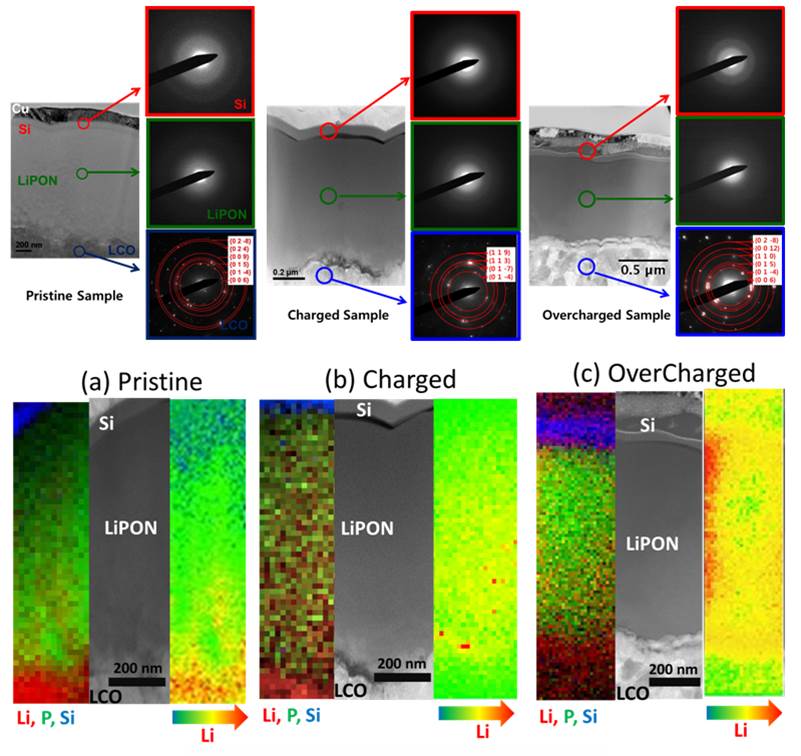
In addition to its thin film work, the Meng lab is also equipped with bulk all-solid-state lithium battery expertise. Because solid-state electrolytes (SSEs) are non-volatile and non-flammable, bulk all-solid-state batteries can endure much harsher conditions than a conventional Li-ion battery without the fear of catastrophic failure. This attribute makes solid-state batteries particularly well suited for transportation applications. To provide a practical energy density (Wh kg-1) for such applications, bulk all-solid-state batteries are nearly an order of magnitude thicker than thin-film batteries. The primary SSE for thin-film batteries, Li3+xPO4-xNx (LiPON), has too low of an ionic conductivity for use in bulk all-solid-state batteries because its ionic conductivity is only 2.0 x 10-6 S cm-1 at room temperature. For this reason, the Meng lab’s expertise primarily entails the synthesis, densification, and characterization of state-of-the-art SSEs with ionic conductivities exceeding 10-3 S cm-1 at room temperature.
Synthesis techniques at our disposal include mechanochemical planetary ball milling, melt-quenching, and high temperature solid-state reactions under both inert and vacuum environments. Regarding the densification of SSEs, the Meng lab has access to a spark plasma sintering machine as well as a hot isostatic press. Finally, the electrochemical properties of SSEs can be measured using standard electrochemistry equipment (Arbin and Solartron) and special Ti-PEEK test cell dies. The synthesis of each class of SSE comes with its own unique set challenges. For example, oxide ceramics like Li7La3Zr2O12 (LLZO) and Li1-x-yAlxMyM2-x-y(PO4)3 (LAMP) must be sintered in order to minimize interfacial grain boundary impedance. On the other hand, Li2S-P2S5 based glass-ceramics have very low interfacial grain boundary impedance. Unfortunately, sulfide based electrolytes tend to be extremely hydroscopic and must be handled only in inert environments. Anti-perovskites inherit the challenges of both the oxide and sulfide SSEs.
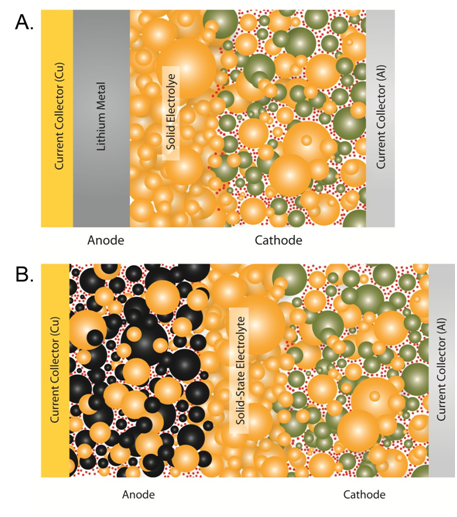 A) Bulk all-solid-state lithium metal battery. B) Bulk all-solid-state Li-ion battery. Battery components include glass-ceramic electrolyte (orange), conductive additive (red dots), cathode active material (green), and anode active material (black)
A) Bulk all-solid-state lithium metal battery. B) Bulk all-solid-state Li-ion battery. Battery components include glass-ceramic electrolyte (orange), conductive additive (red dots), cathode active material (green), and anode active material (black)
Zinc-air Fuel Cell
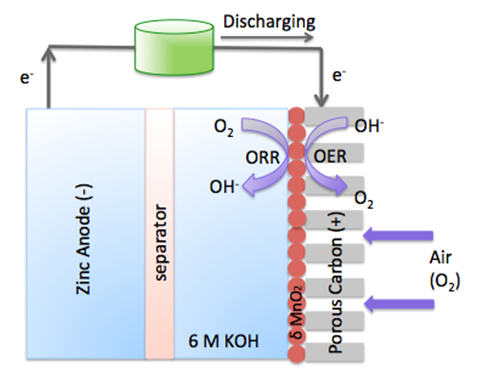 The zinc air fuel cell is a promising energy storage cell because of its high specific energy, aqueous and nonflammable electrolyte, and zinc’s abundance. A functional zinc air fuel cell is composed of three parts: 1) zinc metal as the anode; 2) separator; and 3) an air electrode as the cathode. In this cell the cathode contains a gas diffusion layer and a catalyst layer. Since the oxygen reaction is sluggish, it is important to find a suitable catalyst that can accelerate this reaction. Currently, the catalysts used to facilitate both the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) are extremely expensive. Therefore, in order to commercialize this system alternatives such as metal oxides are of specific interest. In this research, we are interested in developing cost-effective, high electro-catalytic, and phase stable oxide catalyst which can enhance the OER and ORR. Furthermore, we are also focusing on the characterization and mechanism of both the zinc air fuel cell and catalyst.
The zinc air fuel cell is a promising energy storage cell because of its high specific energy, aqueous and nonflammable electrolyte, and zinc’s abundance. A functional zinc air fuel cell is composed of three parts: 1) zinc metal as the anode; 2) separator; and 3) an air electrode as the cathode. In this cell the cathode contains a gas diffusion layer and a catalyst layer. Since the oxygen reaction is sluggish, it is important to find a suitable catalyst that can accelerate this reaction. Currently, the catalysts used to facilitate both the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) are extremely expensive. Therefore, in order to commercialize this system alternatives such as metal oxides are of specific interest. In this research, we are interested in developing cost-effective, high electro-catalytic, and phase stable oxide catalyst which can enhance the OER and ORR. Furthermore, we are also focusing on the characterization and mechanism of both the zinc air fuel cell and catalyst.
Magnesium-ion batteries
Magnesium-ion batteries have been proposed as a potential alternative to Li-ion secondary electrochemical cells. Advantages include lower cost and higher abundance of Mg than Li as well as a higher theoretical specific volumetric capacity compared to Li. However, many problems preclude the widespread use of this new technology. These include poor cyclability, low voltage-stability window compared to Li, and use of complex, expensive electrolytes. To mediate these issues, optimization of Mg-ion battery components has become a hotbed of scientific research. Much of the difficulty in Mg-ion systems that utilize Mg metal as an anode is that a passivation layer forms on the Mg surface, preventing deposition of Mg ions. Therefore, research has been directed at preventing or circumventing this layer.
We are currently working to mitigate the aforementioned problems by developing state-of-the-art organic and inorganic cathodes as well as utilization of various electrolytes. For instance, intercalation materials beyond Chevrel-phase Mo6S8 are being tested for improved specific capacity and cyclability. This includes highly porous zeolithic type materials as well as conventional intercalation materials. In addition, recent studies have exemplified the use of non-conventional electrolytes to achieve reversible deposition/dissolution of electroactive Mg-ions.
To fully characterize our novel systems, we utilize a number of different techniques. This includes SEM, optical microscopy, XRD, FTIR, as well as various electrochemical experiments.
Highlighted Publications
J. Xu, D. H. Lee, Y. S. Meng, “Recent advances in sodium intercalation positive electrode materials for sodium ion batteries“, Functional Material Letters, 2013, 6, 1330001
D. H. Lee, J. Xu, Y. S. Meng, “An advanced cathode for Na-ion batteries with high rate and excellent structural stability“, Phys. Chem. Chem. Phys., 2013, 15, 3304
M. G. Verde, K. J. Carroll, Z. Wang, A. Sathrum, Y. S. Meng, “Achieving high efficiency and cyclability in inexpensive soluble lead flow batteries“, Energy & Environmental Science, 2013, 6, 1573
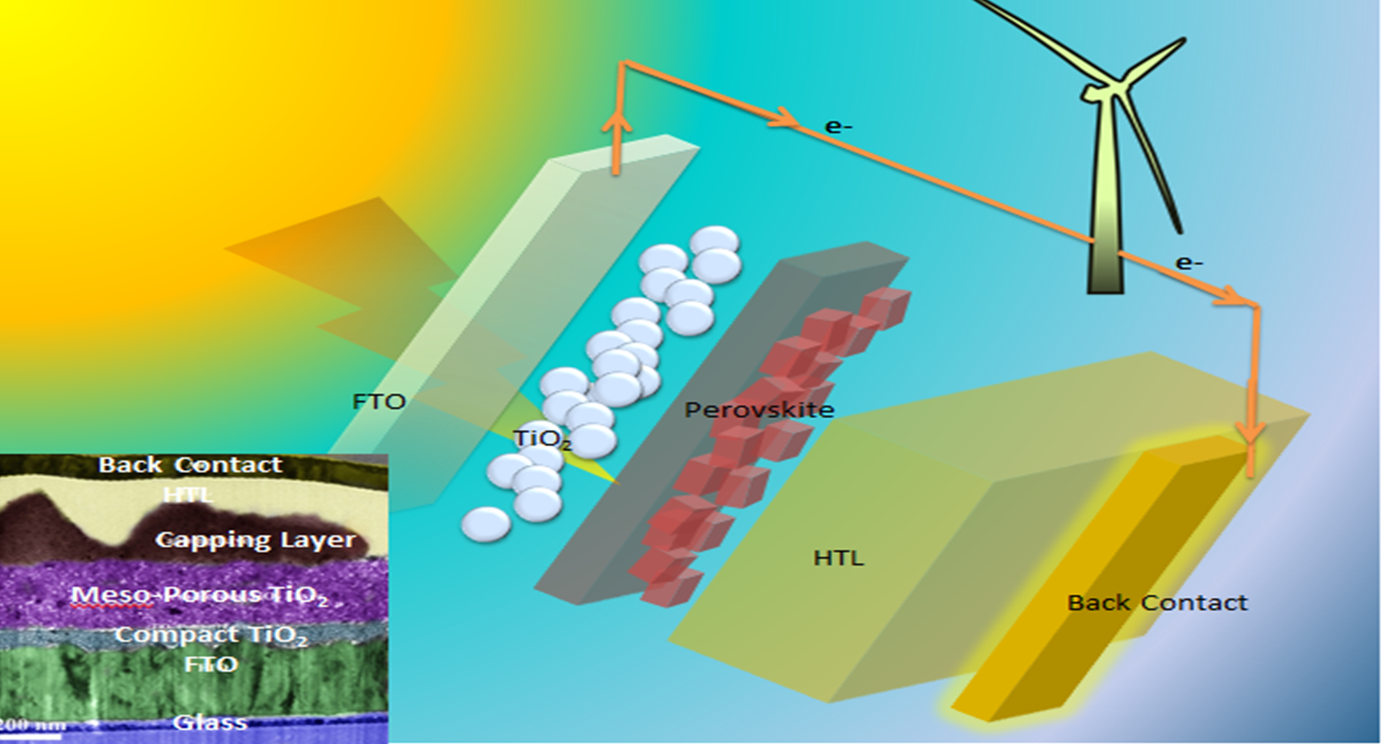
Hybrid organic–inorganic methylammonium lead halide perovskites, are recognized for their excellent semiconducting properties. These novel materials have been applied as the intrinsic layer in perovskite solar cells
As the third generation of solar cells, perovskite solar cells (PSCs) have high power-conversion efficiency. The fundamental charge generation and transportation mechanisms for PSC are similar to that of dye-sensitized solar cells.
Our research is focusing on the working mechanism and degradation process of PSCs. With the help of advanced characterizations like FIB-TEM, APT, and STEM-EELS, we can deeply understand their charge transfer and recombination mechanisms.
Highlighted Publications
S. Wang, M. Sina, P. Parikh, T. Uekert, B. Shahbazian, A. Devaraj, and Y. S. Meng, “Role of 4-tert-Butylpyridine as a Hole Transport Layer Morphological Controller in Perovskite Solar Cells” Nano Letters, 2016, 16, 5594
S. Wang, W. Yuan, and Y. S. Meng. “Spectrum-Dependent Spiro-OMeTAD Oxidization Mechanism in Perovskite Solar Cells“, ACS Appl. Mater. Interfaces, 2015, 7 (44), 24791
Novel Synthesis builds customized materials for energy storage and conversion
Solid State Thin Films Deposited by Pulsed Laser Deposition
 Synthesized thin film lithium ion battery materials present many advantages for characterization and performance over the conventional slurry electrodes made for coin cell batteries. The absence of conductive and binder additives facilitates the direct reaction with the active material. This is crucial in surface characterization techniques where additives could convolute the signal from the active material. Solid state battery full cells also offer advantages over liquid cells, in safety and energy density. Unlike liquid organic electrolytes, solid state electrolytes used in solid state batteries are not sensitive to flammable conditions. Advanced analytical characterizations like in-situ TEM are enabled by solid state batteries. The small dimensions of the components and solid nature ease the fabrication of nano-batteries that can be observed under TEM. Therefore, solid state thin films are of great interest in fundamental studies of lithium ion battery materials which can be used in various applications. Our thin films are deposited with a Lambda Physik LPX-Pro 210 laser along with a high vacuum chamber with a substrate heater.
Synthesized thin film lithium ion battery materials present many advantages for characterization and performance over the conventional slurry electrodes made for coin cell batteries. The absence of conductive and binder additives facilitates the direct reaction with the active material. This is crucial in surface characterization techniques where additives could convolute the signal from the active material. Solid state battery full cells also offer advantages over liquid cells, in safety and energy density. Unlike liquid organic electrolytes, solid state electrolytes used in solid state batteries are not sensitive to flammable conditions. Advanced analytical characterizations like in-situ TEM are enabled by solid state batteries. The small dimensions of the components and solid nature ease the fabrication of nano-batteries that can be observed under TEM. Therefore, solid state thin films are of great interest in fundamental studies of lithium ion battery materials which can be used in various applications. Our thin films are deposited with a Lambda Physik LPX-Pro 210 laser along with a high vacuum chamber with a substrate heater.
Hydrothermal Synthesis
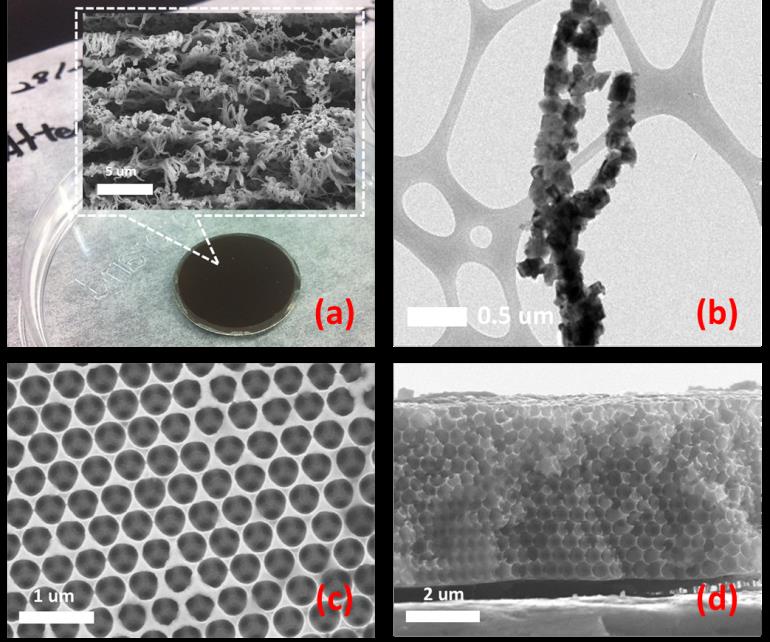
 Hydrothermal synthesis can be defined as a method of synthesis of crystal nucleation which is dependent on the solubility of the precursors under high temperature and pressure. The temperature gradient is maintained inside a Teflon container, where at a high temperature regime the solute dissolves and at a low temperature regime crystal is deposited which can be grown to the desired size. This is particularly suitable for the synthesis of crystallized particles with different morphologies. As shown in the figure: (a, b) spherical secondary particles, (c, d) square secondary particles. LESC utilizes this method to synthesize cathode materials for lithium ion batteries. Different cathode material morphologies can be synthesized to improve the electrochemical performance of a battery. LESC can also utilize specific tools (in advanced characterization) to further optimize battery electrodes synthesized using the hydrothermal process.
Hydrothermal synthesis can be defined as a method of synthesis of crystal nucleation which is dependent on the solubility of the precursors under high temperature and pressure. The temperature gradient is maintained inside a Teflon container, where at a high temperature regime the solute dissolves and at a low temperature regime crystal is deposited which can be grown to the desired size. This is particularly suitable for the synthesis of crystallized particles with different morphologies. As shown in the figure: (a, b) spherical secondary particles, (c, d) square secondary particles. LESC utilizes this method to synthesize cathode materials for lithium ion batteries. Different cathode material morphologies can be synthesized to improve the electrochemical performance of a battery. LESC can also utilize specific tools (in advanced characterization) to further optimize battery electrodes synthesized using the hydrothermal process.
The Polyol Process
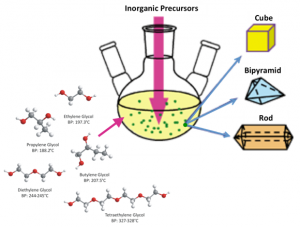 Among the chemical, physical, or electrochemical process generally used in particles production the polyol synthesis of inorganic nanoparticles is simple and versatile. The polyol process allows one to control the nucleation and growth steps in specific media. This process yields particles with well-defined characteristics, uniform shape, submicron or nanometer range with a narrow size distribution, and with a low degree of agglomeration.
Among the chemical, physical, or electrochemical process generally used in particles production the polyol synthesis of inorganic nanoparticles is simple and versatile. The polyol process allows one to control the nucleation and growth steps in specific media. This process yields particles with well-defined characteristics, uniform shape, submicron or nanometer range with a narrow size distribution, and with a low degree of agglomeration.
This is a facile route for preparing finely divided metal powders by reduction of inorganic precursors in liquid polyols. The solid precursor is suspended in the liquid polyol, which may be quite soluble (nitrate, chloride, acetate) or only slightly soluble (oxide, hydroxide). The solution or the suspension is stirred and heated at a specific temperature which reaches the boiling point of the polyol. Using this method of synthesis has several advantages such as high permittivity, mild reducing power conditions, and good chelating properties.
Nano-Structured Electrode Fabrication
Nano-structured materials for lithium-ion batteries have various advantages compared to the conventional micrometer-sized particles. The short distances for the lithium-ion transport within the materials increases significantly the rate of (de)intercalation. The characteristic time constant for diffusion is given by t=L2/D, where L is the diffusion length and D the diffusion constant. The time t for intercalation decreases with the square of the particle size on replacing micrometer with nanometer materials. In addition, a high surface area permits a high contact area with the electrolyte and hence a high lithium-ion flux across the interface. Electron transport with the materials is also enhanced by nanometer-sized materials. With the help of sol-gel based template synthesis, LESC can successfully fabricate the one-dimensional nano-structured cathode electrodes (a,b). The template-assisted electrodepostion with a self-assembled opal template also enable to obtain the three-dimensional nano-structured anode electrodes (c,d). LESC utilizes these synthesis methods to study the quantitative understanding of the effect of the nano-structural geometry on the elementary reaction steps involed in their electrochemical operation.
HIGHLIGHTED PUBLICATIONS
K. Carroll, M. Yang, G. M. Veith, N. J. Dudney, Y. S. Meng, “Intrinsic surface stability in LiMn2-xNixO4-δ (x=0.45, x=0.5) high voltage spinel materials for lithium ion batteries“, Electrochemical and Solid State Letters, 2012, 15(5), A72
H. Xia, Y. S. Meng, M. O. Lai, L. Lu, “Structural and Electrochemical Properties of LiNi0.5Mn0.5O2 Thin-Film Electrodes Prepared by Pulsed Laser Deposition“, Journal of the Electrochemical Society, 2010, 157 (3), A348
H. Xia, Y. S. Meng, M. O. Lai, L. Lu, “Structural and Electrochemical Properties of LiNi0.5Mn0.5O2 Thin-Film Electrodes Prepared by Pulsed Laser Deposition“, Journal of the Electrochemical Society, 2010, 157 (3), A348
H.-M. Cho, Y. S. Meng, “Effect of Ni/Mn ordering on elementary polarizations of LiNi0.5Mn1.5O4 spinel and its nanostructured electrode“, Journal of the Electrochemical Society, 2013, 160(9), A1482
Advanced Characterization provides insightful understanding of nanomaterial.
X-ray Photoelectron Spectroscopy
Quasi in-situ X-ray photoelectron spectroscopy (XPS) is utilized to examine surface transformations of the cathode electrode during electrochemical cycling. LESC utilizes a quasi in-situ setup where samples can be charged to different states and analyzed without air exposure.XPS can provide information on elemental identification, chemical state of element, and relative composition of constituents in surface region. This is critical for the development of new battery materials.
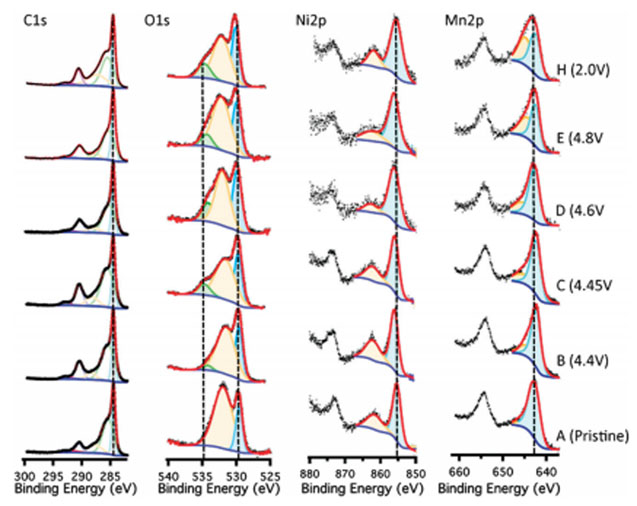
This figure shows the resulting XPS spectra for the C1s, O1s, Mn2p and Ni2p region scans at various points during the first electrochemical charge and discharge. For example,the pristine Ni2p shows a binding energy of 855 eV. The peak shifts to a higher binding energy of 856.5 eV when fully charged, suggesting that the nickel oxidation state change is complete. Upon discharge the peak shifts back to the Ni2+.
Neutron Diffraction
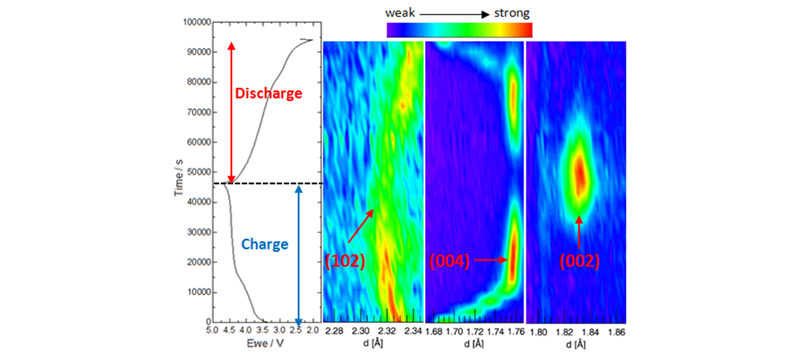
In-situ neutron diffraction (ND) has been developed to study the crystal structure evolution of both the cathode and the anode in a full cell during electrochemical cycling. The advantages of neutron diffraction: 1) high penetration enables simultaneous observation of cathode and anode during charge and discharge; 2) high sensitivity to light elements, lithium and oxygen; and 3) high contrast to distinguish TM ions. With the help of in-situ neutron diffraction, we can observe the phase transformation of graphite, lithium intercalation mechanism and oxygen evolution of the cathode material.
(a): 1st cycle charge/discharge curve of Lithium-rich / C cell and surface contour plots of (b): Lithium-rich (102); (c): LixC6 (004); (d): LiC6 (002) Neutron Diffractions.
Synchrotron X-ray Diffraction
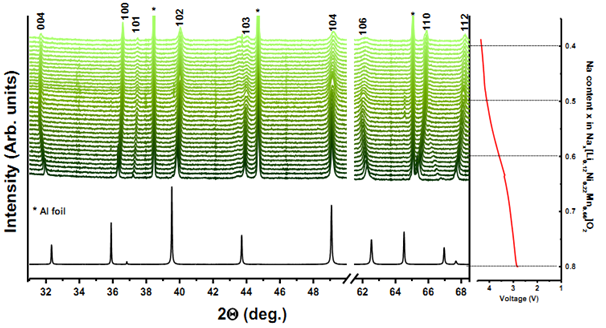 Due to the merits of high brightness and high intensity, high level of polarization and wide tunability in energy, synchrotron radiation technique provides an unique platform for analysis of the relationship among composition-structure-performance of materials for electrochemical system. Both ex-situ and in-situ X-ray Diffraction (XRD) data are collected by our group with a variety of electrode materials at Argonne National Lab on beamline 11-BM and at Brookhaven National Lab on beamline X14A respectively. The ex-situ studies of active materials involve arresting the electrochemical cycling at various stages and extracting the materials for characterization, so it may fail to capture short-lived metastable phases. The development of in-situ XRD with synchrotron sources further enables to monitor the phase evolution upon cycling and analyze the atomic site occupancy at the same time.
Due to the merits of high brightness and high intensity, high level of polarization and wide tunability in energy, synchrotron radiation technique provides an unique platform for analysis of the relationship among composition-structure-performance of materials for electrochemical system. Both ex-situ and in-situ X-ray Diffraction (XRD) data are collected by our group with a variety of electrode materials at Argonne National Lab on beamline 11-BM and at Brookhaven National Lab on beamline X14A respectively. The ex-situ studies of active materials involve arresting the electrochemical cycling at various stages and extracting the materials for characterization, so it may fail to capture short-lived metastable phases. The development of in-situ XRD with synchrotron sources further enables to monitor the phase evolution upon cycling and analyze the atomic site occupancy at the same time.
Scanning Transmission Electron Microscope and Electron Energy Loss Spectroscopy
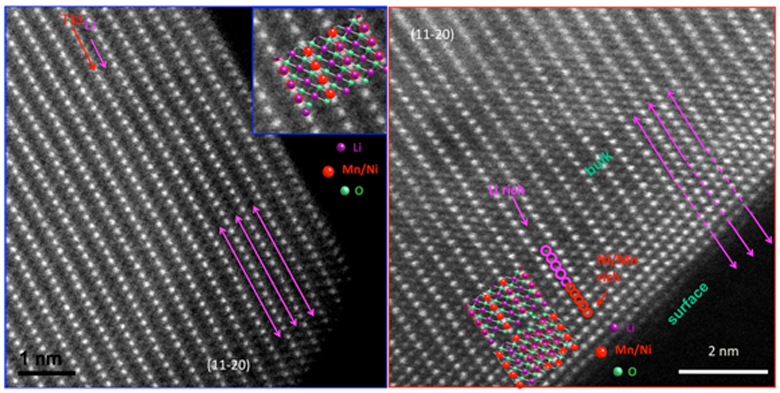 Scanning Transmission Electron Microscope and Electron Energy Loss Spectroscopy is a powerful tool that can obtain structural and chemical information with atomic resolution. The annular dark/bright field detector coupled with a spectrometer can simultaneously provide the local image and chemical information. LESC has applied this high-end technique to battery systems in ex-situ and in-situ, achieving both electrode and interface properties.
Scanning Transmission Electron Microscope and Electron Energy Loss Spectroscopy is a powerful tool that can obtain structural and chemical information with atomic resolution. The annular dark/bright field detector coupled with a spectrometer can simultaneously provide the local image and chemical information. LESC has applied this high-end technique to battery systems in ex-situ and in-situ, achieving both electrode and interface properties.
Coherent X-ray Diffractive Imaging
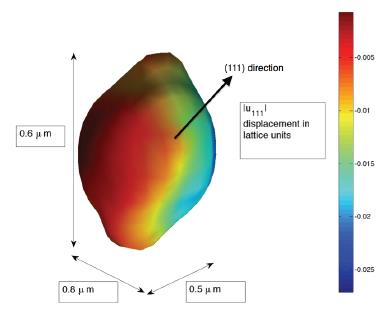
Coherent X-ray diffractive imaging (CXDI) is a three-dimensional structural analysis method for individual small crystals. A spatially coherent beam of X-rays traverse the sample, and produce the scattering from all parts of the sample, which can be expected to interfere in the far-field diffraction pattern. With the help of the computational phase retrieval algorithms, the inversion of the diffraction pattern yields a three-dimensional image of the density distribution and a projection of the strain within it. LESC utilizes this characterization to map the local three-dimensional strain inhomogeneity and electron density distribution of the battery materials in both ex-situ and in-situ methods. This will shed a new light on understanding the nano-mechanics and phase transformations during the battery operation.
In-Situ Transmission Electron Microscope
HIGHLIGHTED PUBLICATIONS
H. D. Liu, C. R. Fell, K. An, L. Cai, Y. S. Meng, “In-situ neutron diffraction study of the xLi2MnO3-(1-x)LiMO2 (x=0, 0.5; M=Ni, Co, Mn) layered oxide compounds during electrochemical cycling“, Journal of Power Sources, 2013, 240, 772
J. Xu, D. H. Lee, R. J. Clement, X. Yu, M. Leskes, A. J. Pell,G. Pintacuda, X.-Q. Yang, C. P. Grey, Y. S. Meng, “Identifying the Critical Role of Li Substitution in P2-Nax[LiyNizMn1-y-z]O2″, Chem. Mater., 2014, 26, 1260
D. Santhanagopalan, D. Qian, T. Mcgilvary, Z. Wang, F. Wang, F. Camino, J. Graetz, N. Dudney, Y. S. Meng, “Interface Limited Lithium Transport in Solid-State Batteries“, J. Phys. Chem. Lett., 2014, 5, 298
A. Ulvestad, H. M. Cho, R. Harder, J. W. Kim, S. H. Dietze, E. Fohtung, Y. S. Meng, O. G. Shpyrko, “Nanoscale strain mapping in battery nanostructures“, Applied Physics Letters., 2014, 104(7), 073108
Computation and Simulation provide us with a map which can be applied to nanomaterials.
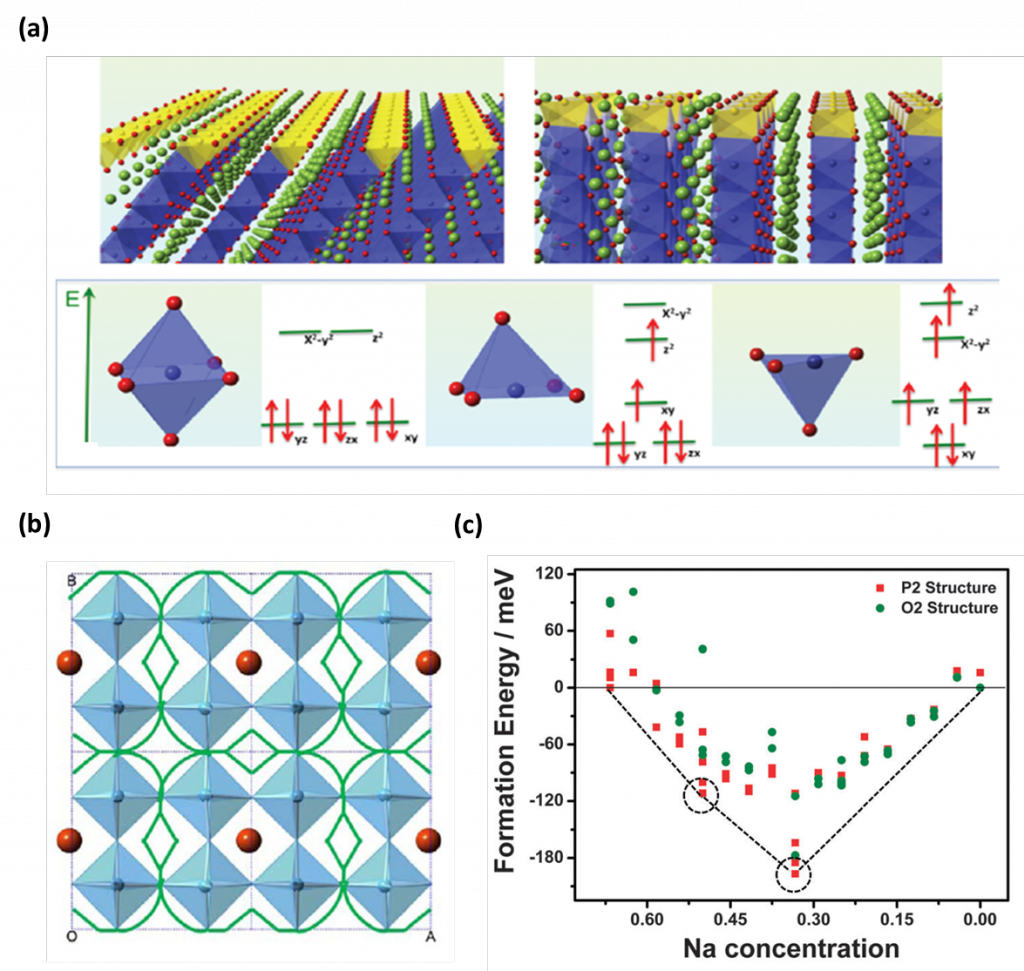
The important characteristics of rechargeable battery materials including electronic properties, ions path and their diffusivities, and the structural stability can be investigated state-of-the-art first principles calculations using VASP package. For example, Figure 1a. shows electronic spin transition from low spin to high spin in the surface of nanosized lithium cobalt oxide during (de)intercalation 1. This work suggests the significant criteria to develop high functional electrode materials by controlling the surfaces and interfaces of nano-sized materials. In addition, Li-ion diffusion path can be estimated by calculating its activation barriers suggesting Li-ion prefer a curved diffusion path to avoid empty sites as shown in Figure 1b 2. Thermodynamically the most stable structures in certain concentration of ions can also be estimated with calculated convex hull of formation energies as shown in Figure 1c 3. This is the direct measure of phase stability and identifies the intermediate phases during electrochemical reactions
Figure 1. (a) Electronic spin transition from low spin to high spin in the surface of nano-sized LiCoO2, (b) estimated Li diffusion path in La-poor layer, and (c) the formation energy convex hull (dotted line) for Nax[Ni1/3Mn2/3]O2 cells.
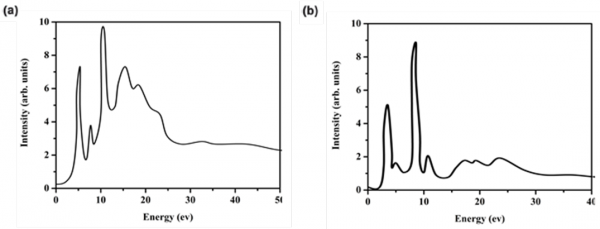
WEN2K package is also applied in our lab in order to fully understand the fine structures of electron energy loss spectroscopy (EELS). The key fact about first principles calculations WIEN2K package is that the core shell electrons and the valence electrons are treated distinguished, a linear combination of radial wave functions multiplied by spherical harmonics used for the core electrons, a plane wave expansion used for the valence electrons. This feature makes the WIEN2K give more accurate simulation of the EELS result compared with other computational package based on the pseudopotential method like VASP package as shown in Fig. 2. Our objective is to explore the detailed local electronic structures of lithium cathode materials, and simulate the EELS spectra based on the obtained density of states. We could also adopt the same theoretical and computational method to other more complex systems, such as core-shell structure and cathode materials with gradient structure.
Figure 2. (a) Lithium EELS K edge simulation using WIEN2K package for LiMn2O4, and (b) Li2O.
HIGHLIGHTED PUBLICATIONS
D. Qian, B. Xu, H.-M. Cho, T. Hatsukade, K. J. Carroll, Y. S. Meng, “Lithium Lanthanum Titanium Oxides: A Fast Ionic Conductive Coating for Lithium-Ion Battery Cathodes“, Chemistry of Materials, 2012, 24(14), 2744
D. Qian, Y. Hinuma, H. Chen, L-S Du, K. Carroll, G. Ceder, C. P. Grey, Y. S. Meng, “Electronic spin transition in nanosize stoichiometric lithium cobalt oxide“, Journal of the American Chemical Society, 2012, 134(14), 6096
D. H. Lee, J. Xu, Y. S. Meng, “An advanced cathode for Na-ion batteries with high rate and excellent structural stability“, Phys. Chem. Chem. Phys., 2013, 15, 3304
Current Funding
Battery 500 Consortium

Ford Motor – University Research Program (URP)

Maxwell Technologies
California energy commission
Batteries for Advanced transportation technologies

Single Investigator & Small Group Research (SISGIR)
Energy Frontier Research Center (EFRC)
DoD SBIR/STTR
Advanced Research Project Agency – Energy
National Science Foundation
Air Force Office of Scientific Research
Past Funding
CheMat
NEI Corporation
NASA
General Atomics
VIRGINIA COMMONWEALTH UNIVERSITY (VCU)
AMERICAN CHEMICAL SOCIETY PETROLEUM RESEARCH FUND